You are here
Search by issue
Media Releases & News
- SU Media Releases
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
- 2016
- 2015
- 2014
- 2022
- 2013
- 2012
- 2011
- 2010
- 2009
- General Statements
- Sovereign Treaties: International Law
- Walkout Statement from Sacred Fire
- Proclamation (with video Readings)
- UDI's Explained
- What is 'Decolonisation'?
- Uluru Statement from the heart
- Sovereign Manifesto of Demands
- NO CONSENT to Recognition
- Submission on ILUA Amendment
- Uluru Statement video testimonials
- Uluru Statement grassroots videos
- Complexity of Treaty and Treaties
- Understanding Treaty & Treaties
- Official SU Meetings
Sovereign Union Menu
- Self Governance
- International
- Treaty - Treaties
- Sovereignty
- General Principles of Sovereignty
- First Nations sovereignty defined
- The Sovereignty Debate
- Sovereignty into Governance
- Reform: Practicing sovereignty
- Sovereignty after Mabo
- What about Sovereignty?
- Current sovereignty debate
- Sovereignty protest Qld
- Research papers & Theses
- Sovereignty never ceded -thesis
- SA sovereignty challenged
- Rosalie Kunoth-Monks leads the way
- Pacific Islanders Act 1875
- Asserting Aboriginal Sovereignty
- Aboriginal rights then & now
- Michael Kirby Oration
- Academic Paper : Songlines
- John Howard recognised sovereignty
- Queen Victoria: Crown owns nothing, Aborigines sovereign
- Rejecting 'recognition'
- Yingiya Mark Guyula Speach
- New Way Summits
- Decolonise
- August
- Australia: UN Questionnaire
- July
- Law against Genocide Bill
- Rally: 'Let us go Home'
- Ray Jackson: 30 years of light
Special Features
- History
- During Invasion
- Invasion
- 1788-2014 Invasion to Resistance
- 'The condition of the natives' 1905
- Mapping QLD massacres
- Cooks Instructions & Diary
- Whites & Blacks during the colonisation in the late 19th Century
- Australia's history of slavery
- Dad 'n Dave - The Qld killing fields
- Treatment of Aboriginal Prisoners
- 'Conspiracy of silence' blood baths
- British Parliament Report 1837
- Culture
- Horrors of North West WA
- Ignorance & Racism
- Invasion
- Post Invasion
- Stolen Remains and Collections
- Culture
- Kitty Wallaby: Dreamtime & colanisation
- Improve heritage management
- Unlock secrets of rock art
- Kurlpurlunu found in Central Australia
- Forgotten' Woolwonga tribe for 130 years
- First Nations rock art is at risk
- Language diversity threatened, study
- Languages Treasure trove
- Pressure to lose values and culture
- Languages reveal scientific clues
- Ignorance & Racism
- Father of the Stolen Generation
- Aboriginal counting myth won't go away
- Pleading letters to 'Protector'
- 1926 plan for an Aboriginal state
- First prisoner abuse inquiry 1905
- Legalised slavery: hidden reality
- The second dispossession
- Rottnest Island internment camp
- Meston's 'Wild Australia' Show 1892-1893
- Stolen Wandjina: Cultural Appropriation
- Whitefellas destroyed our bible
- Fraser Island Death Camp
- Intervention to kill self-determination
- Ongoing Warfare
- Pre Invasion
- Culture
- Genesis
- Out of Australia - Not Africa
- confirmation of genetic antiquity
- WA's Mid West History 30K
- 'Australian' peoples were first Americans
- How First Nations saw the Stars
- Ice Age struck First Nations people hard
- Kimberley paintings could be oldest
- Misunderstand First Nations science
- Sea-Level recorded
- James Cook's Secret Instructions - 30 June 1768
- Trading & Hospitality
- Landcare, Science, Agriculture
- Captain Cook
- During Invasion
- The Frontier Wars
- Memorial Marches
- The Frontier Wars
- Anzac Gathering 2012
- Aboriginal servicemen honoured
- 1905 Report WA's North
- Anzac Day Videos
- Battle for FF memorial
- From invasion to resistance
- Frontier Wars Remembered 2016
- Image Galleries
- Invasion & Sovereignty
- Invasion Day Callout for 26 January
- Memorial ignores frontier war
- The Freedom Fighters
- Truth about the Gulf country
- Conflicts/Massacres
- Massacres & Trophies
- Mixed Media
- Canberra 2016
- Closing the Homelands
- Homelands explained
- Central Articles
- Closing Homelands for mining
- Vital for ecosystems
- Terra nullius never went away
- Barnett's plan to axe homelands for years
- Fed Gov't $100 million deal with states
- Mining: $10,000 job sweeteners
- Abbott rejects international law
- Barnett's War on Health
- Anatomy of Racism in 2015
- Food supply autonomy needed
- Freedom Summit: WFD Media Release
- Funding: Confusing-fractured-racist
- It's OK to discriminate
- The demise of Coonana community
- Sovereign Union Articles
- Reports by State & Territory
- Video - Images - Audio
- Rallies
- Media Reports
- Homeland Closures
- 'Recognition' Constitution
- Nuclear Waste Dumps
Key Topics & Issues
- NT Detention Hearings
- NT Intervention
- Sovereign Embassies
- All Embassies - Map
- Embassies
- Canberra Tent Embassy
- Perth Sovereign Embassies
- Portland Sovereign Embassy
- Brisbane Sovereign Embassy
- Brisbane Embassy - Home
- What is Brisbane Embassy?
- 2013 content
- 2012 content
- New Stolen Generation rally
- Police arrest sovereigns
- Politically motivated eviction
- Brisbane - Eviction notice
- Brisbane Embassy meets CMC
- Brisbane conference 2012
- Charges dropped but ...
- Council meets Elders
- Embassy arrestees protest
- Embassy midnight raid
- Embassy to be shut down
- Fire destroys Embassy
- Musgrave heritage listing
- Sovereigns charges dropped
- Moree Sovereign Embassy
- Broome Tent Embassty
- Woomera Sovereign Embassy
- Airds Sovereign Embassy
- Gugada Sovereign Embassy
- Tent Embassy in Airds, Campbelltown
- Call for Embassy tolerance
- Embassy Articles & Media
- Land, Sea & Water
- Northern Murray Darling Basin
- Water Treaty Talks: Murray-Darling Basin Nations
- Mining Country
- Adnyamathanha people of the rocks
- Ancient approach to global emissions
- Danger: Developing the North
- First Nations Land Management
- Historical sites in NSW
- Clark and Mansell: trading beef
- David Suzuki: Aboriginals best bet
- Dingoes may save wildlife
- Fires support mammals - research
- First Nations had villages and farmed
- Homelands vital for fragile ecosystems
- Hunter gatherers Myth - Bruce Pascoe
- Kangaroos win after hunt with fire
- Race to protect ancient rock art
- Kakadu Park 40th Anniversary
- Land rights and the government
- Native 'wild' rice in Australia
- Native Title amendments slammed
- Ngoongar Grower group using Native Youlks
- WA Aboriginal Heritage Act overview
- Sovereign Neighbours
- West Papua - Freedom Flotilla
- Articles - Free West Papua Movement
- December
- October 2013
- September 2013
- Emergency Protest of Illegal Deportation
- FF activists a test for Abbott government
- West Papuans Deported to Port Moresby
- Six West Papuan's flee after ceremony
- Freedom Flotilla claims success
- Sacred water and fire celebrated
- Ceremony completed in secret
- Military buildup in destination port
- Men released: Treason charges pending
- Julie Bishop incites military action
- Threats to turn back boat
- Four arrested at FF Welcoming
- Time for human rights in Papua
- August 2013
- Media Files
- Articles - Free West Papua Movement
- Atooi Unification Ceremony
- Atooi now has jurisdiction
- Australia & Fiji union
- Canada Day: Time of Mourning
- Canada First Nations alliance
- Canada: Commercial fishing rights
- India: Ruling against mining co
- Indigenous rights struggle
- North American Indians seek Sovereignty
- Statue honors US freedom fighters
- The Creation of one world gov't
- Vanuatu: Pacific custom land tenure
- Arizona tribal law system
- Canada: Tsilhqot'in peoples win ruling
- Embassy meets Indonesians
- Grandmothers: worst ever stealing
- Ibans acquired native customary rights
- Maori did not cede sovereignty
- UN: Canadian Aboriginals in crisis
- West Papua - Freedom Flotilla
- The Invasion
- A day of sadness
- Health and Social Issues
- Suicides & Depression
- Suicides just happened?
- Nothing will be done about the suicides
- First Nations suicide rates
- Gov't not listening - People die
- 77 suicides in SA alone
- 996 deaths by suicide
- Hundreds will suicide if we wait
- Suicides: 'A humanitarian crisis'
- Suicide is humanitarian crisis
- Suicide epidemic worldwide
- Noongar x3 suicide rate
- Falsely arrested & drugged
- Cashless Welfare Card
- Family breakdowns causing repeats
- General Health Issues
- Government Policies
- Youth suicide at crisis point
- Negatively framing policies
- A little boy who hid under the bed
- Defend and Extend Medicare
- Call for help results in children stolen?
- Doctors in communities in WA reduced
- Children and human rights
- Our children more likely to be removed
- Justice? wake up WA
- Need for Bilingual schools
- Racism Effects
- Rosie Fulton
- Work-for-the-Dole Unhealthy
- Youth lost in prisons: Amnesty
- Suicides & Depression
- Gross Abuse
- Genocide and apartheid
- National
- States & Territories
- Suicides
- Imprisonment & Deaths
- International Critisism
- Genocide
- More Gov't Abuses
- Mining and Destruction
- Racism
- Racism Game in Australia
- 27% say it's OK to be racist
- Abbott backs out of Racism changed
- Genocide by Apartheid Australia
- How Bolt should be punished
- Racial discrimination in Australia
- Racism Media
- Racism driver for ill health
- Racism is a health issue
- Racism worse than Sth Africa
- Reform: Just Ask Black Australia
- Unpacking White Privilege
- Welcome to Dja Dja Wurrung Country
- White Australian National Anthem
- Solidarity
Media & Resources
Activism and Politics
- Activism
- Homelands
- For the record: Sovereignty Never Ceded
- Freedom Fighters memorial call
- Anthony Fernando 1864–1949
- Fernando's Ghost - Transcript
- Historic Videos and Images
- Australia’s dirty secret
- Deebing Creek development
- Pilbara pastoral strike
- Pro-Black Isn't Anti-White
- Truth Telling Uluru Statement
- UN slams anti-protest laws
- Whitlam: Legacy of Wave Hill
- Winyirin Bin Bin, Pilbara Strike
- Politics
Kidney disease in Aboriginal Australians perpetuates poverty

(Image: transplant.org.au)
Roger Smith & Kirsty Pringle
The Coversation 10 June 2013
The recent death of the lead singer of Yothu Yindi, is a high-profile example of an event all too common in Aboriginal Australia.
Older Aboriginal Australians (40 to 60 years old) are more than 15 times more likely to die of kidney disease than non-Aboriginal Australians. This is an age that’s normally the prime life. But not only is it a tragedy for the individuals involved but has a much wider effect on the community.
Elders in all communities are a repository of knowledge and of accumulated wealth. Early death of key older family members deprives younger community members of the benefit of accrued knowledge of culture and both financial and social support.
The structure of the broader Australian population is like a pillar with similar numbers of people in all age groups. This means that a young non-Aboriginal child will often receive support and guidance from two mature adults with back up from four, still-living grandparents.
The population structure in Aboriginal Australia is quite different and is more like a triangle, with many more children than adults and even fewer living grandparents. This means that an Aboriginal child receives support and guidance from far fewer adults.
This pyramid like structure is generated partly by early death of Aboriginal adults from heart disease, diabetes and kidney disease.
Not a great start
Heart disease, diabetes and kidney disease are non-communicable diseases that are strongly influenced by the environment. Increasing evidence suggests that all three begin as the baby is developing in the uterus. This concept is known as fetal programming or the developmental origins of adult health.
Let us explain by using kidney disease as an example to illustrate the concept. Skin sores can become infected with the bacteria known as streptococcus, this type of infection can lead to kidney damage known as glomerulonephritis. This can happen in childhood, and, if it happens to a girl, her kidneys may already be damaged by the time she becomes pregnant.
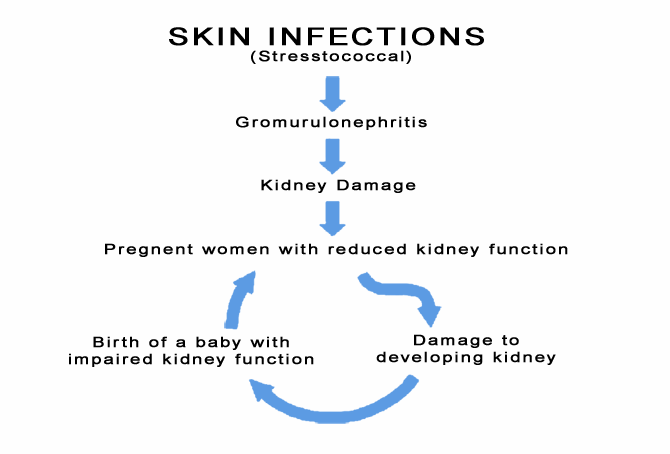
The insidious cycle of kidney disease in the Aboriginal population.
Studies in pregnant sheep have demonstrated that if the mother’s kidney function is damaged, then the kidneys of the developing fetus also become damaged. This allows kidney damage to be passed across generations.
Studies by others suggest that this is happening to many Aboriginal mothers and their babies. Aboriginal mothers often have evidence of kidney disease already present during pregnancy and Aboriginal babies are frequently born with a much smaller number of nephrons (the functional units of the kidney). Typically around 400,000 while non-Aboriginal babies have over one million.
This reduction in nephron numbers is linked to impaired growth within the uterus of many Aboriginal babies who are born too small (known as growth restriction), twice as often as non-Aboriginal babies.
A better way forward
If we are to close the gap in Aboriginal life expectancy and well-being, we need to focus on the beginning of life inside the uterus. We need to ensure high quality care and support for Aboriginal mothers and their babies.
We need to develop ways of identifying babies at risk of kidney disease early to prevent deterioration of kidney function that could be transmitted across generations into the future.
Progress is being made. In Tamworth, a research team from the University of Newcastle’s department of rural health is recruiting young Aboriginal mums and their children and seeking to identify markers of kidney impairment in urine samples.
In Townsville, a neonatologist is using retinal photographs of newborn babies’ eyes to identify those at risk of kidney disease (the blood vessels at the back of the eye reflect the way the blood vessels in the kidney are also developing).
If we can reduce the burden of kidney disease, we can improve not only the health of Aboriginal Australians but also their cultural and material wealth by allowing more older Aboriginal people to transmit their knowledge and resources to the next generation. Intervening early in life to optimise health is a much more effective strategy than trying to correct accumulated damage in later life.
 Roger Smith
Roger Smith
Director of The Mothers and Babies Research Centre at the Hunter Medicial Research Institute at University of Newcastle
 Kirsty Pringle
Kirsty Pringle
Research Fellow in Reproductive Health at University of Newcastle
Disclosure Statement
Roger Smith receives funding from the National Health and Medical Research Council.
Kirsty Pringle does not work for, consult to, own shares in or receive funding from any company or organisation that would benefit from this article, and has no relevant affiliations.
Acknowledgement: Della Yarnold also contributed to this article.

